
They are so called because ions move toward the electrode of opposite charge. A cation is something that moves down ( Greek: κάτω pronounced kato, meaning "down") and an anion is something that moves up ( Greek: ano ἄνω, meaning "up"). The word ion was coined from Greek neuter present participle of ienai ( Greek: ἰέναι), meaning "to go". Ions are also created by chemical interactions, such as the dissolution of a salt in liquids, or by other means, such as passing a direct current through a conducting solution, dissolving an anode via ionization.
IONIC OXYGEN CHARGE FREE
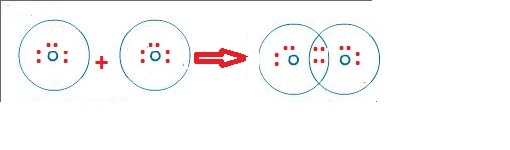
In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a positive ion. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions.

Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.Ī cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. ɒ n, - ən/) is an atom or molecule with a net electrical charge. Forming an ionic bond, Li and F become Li + and F − ions.Īn ion ( / ˈ aɪ. Electron transfer between lithium (Li) and fluorine (F).


 0 kommentar(er)
0 kommentar(er)
